Buying guide: Sodium Formate
If sodium formate is one of the ingredients for your products, then this guide is worth your time.
Read on as we discuss the essentials of sodium formate.
If sodium formate is one of the ingredients for your products, then this guide is worth your time. Read on as we discuss the essentials of sodium formate.

What is sodium formate?
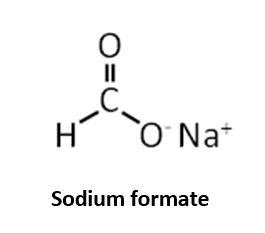
Sodium formate is sodium salt of carbonic acid. It can be used as a chelating agent, and sodium hydroxide is also found in its chemical makeup.
It is known to be an antiseptic agent, which allows it to have sodium hydroxide on its list of uses.
Since sodium formate is only slightly soluble in water, it harnesses the hygroscopic property. This means that the sodium formate absorbs any moisture around it for easy manipulation.
This property is usually applied in manufacturing printed circuit boards and paper products (for better absorption).
How to spot sodium formate: tell-tale signs of sodium formate are placed on products you see every day. Sodium hydroxide might also be added into the formula to help increase its solubility levels.
Chemical properties of sodium formate include:
- Organic sodium compound
- White powder or flakes with sodium hydroxide crystals
- Often found in sodium formate solutions or sodium formate powder
What is sodium formate used for
Aside from being used as an ingredient for sodium formate products, sodium formate has a variety of applications:
- Textile finishers use sodium formate to soften and/or remove excess water. Ferrous solutions are then used to help set dyes and brighten the fabric. This process is called dewatering.
- Extraction of formic acid. Aside from extracting, it helps in the formation of plastics that you see commonly around you today (hard plastics such as polypropylene). This is possible through the reaction between sodium chloride, caustic soda, sodium formate, and oil. – Sodium formate is also used to neutralize sodium hydroxide.
- Used as a cryoprotectant: sodium formate is added into ice cream to prevent it from becoming too hard in production.
- Used in sodium formate soap, sodium formate shampoo, sodium formate conditioner,
- and sodium formate toothpaste.
- Sodium formate and sodium bicarbonate are also used as buffering solutions for manufacturing paper products.
- Sodium formate can be found in toothpaste.
The sodium hydroxide is responsible for converting food debris into simpler substances by breaking down enzymes that cause bad breath. When sodium hydroxide interacts with water present in your saliva, sodium carbonate and sodium bicarbonate are produced which acts as antiseptics good at killing bacteria that cause bad breath.
- Sodium formate is also used as a fire retardant. It can help slow down the fire from spreading quickly and thus giving you time to escape by itself.
- Sodium formate is also a cementing agent for sodium hydroxide and water.
Sodium Formate Solutions
The sodium formate solution that you can buy today has varying concentration levels. 97% of solutions are common in cleaning solution applications.
On the other hand, the weaker forms of sodium formates are most likely used in packaging or printing production.
Using sodium formate for chelation helps improve quality inkjet printing by preventing clogging and drying of ink nozzles on printers.
This gives you a clearer image during photo prints as well as text documents with accurate colors (not faded).
Is sodium formate acid or base?
Sodium formate is a sodium salt of formic acid; sodium formate hydrolyzes in the presence of water to sodium carbonate and HCOOH.
Thus it is an excellent reducing agent, converting into sodium carbonate, which is quite stable and not corrosive.
Sodium carbonate does not have any hazardous characteristics and could be used as a primary reducing agent. It has been widely used in organic synthesis for example.
So you could use sodium formate directly if you want to buy sodium formate for lamination reactions or reduction of carbonyls such as ketones or esters to alcohols.
Is sodium formate toxic?
Sodium formate is corrosive and harmful to the eyes, skin, and respiratory system.
But sodium carbonate which is one of sodium formate’s final products has no toxicity, so sodium formate itself is not very toxic.
Is sodium formate explosive?
The answer is complicated because it depends on temperature and concentration. When the molar ratio of sodium (III) ion/ sodium (II) ion in solution drops to 1:1, sodium metal precipitates out from its sodium salt solutions by a process called auto-decomposition.
Then another major issue appears: how do you separate this highly flammable white solid metal from an inflammable liquid phase containing HCOOH?
So when you want to buy sodium formate as sodium metal, you have to be extra careful about the sodium carbonate in the sodium formate solution.
It is best not to let sodium formate react with sodium carbonate in a strong base or any kind of oxidizer.
Is there sodium formate in sodium bicarbonate?
No. Sodium formate is an acid salt, while sodium bicarbonate (baking soda) is a basic salt, sodium hydroxide being the conjugate base of sodium carbonate (Na 2 CO 3).
They would react with one another to produce sodium hydrogen carbonate and water.
If you want to dissolve sodium metal by sodium carbonate solution, I recommend using liquid sodium bicarbonates or some other cheap chemical-grade sodium formates. They are stable when heated to high temperatures and have low impurity contents.
Is sodium formate natural?
Sodium formate is a sodium salt, so it contains sodium. The purity of sodium formate should be more than 98%.
So when you select sodium formate, you must check the sodium content. Sodium Formate Hydrate (NaHCOO) is sodium formate with hydrate, the sodium content is not more than 0.2%.
Sodium formate price and sodium formate supplier
Sodium Formate is widely used in the metal complex industry, dyeing and printing industry, leather tanning, and other industries, which means there are a lot of sodium formate suppliers now.
But since sodium formate is sodium salt, the sodium content should be controlled strictly, only a small number of sodium formate manufacturers can produce sodium formate with high purity and low sodium content.
This means that you should be careful when selecting sodium formate suppliers and manufacturers.
Sodium formate price is also related to sodium content, if sodium formate suppliers quote sodium formate price too low, you should think twice before you buy sodium from them.
How to buy sodium formate?
When you want to buy China sodium formate, first check your project needs: what kind of grade sodium formate do you need?
Second, check sodium formate cost and sodium formate supplier carefully. If sodium content is too high, your project will be failed.
Finally, when sodium formate arrives at your factory or warehouse in large amounts, you should check sodium formate quality once again to make sure it meets the requirement of your project.
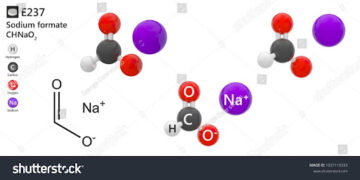

Sodium formate is sodium salt of carbonic acid.
It can be used as a chelating agent, and sodium hydroxide is also found in its chemical makeup.
It is known to be an antiseptic agent, which allows it to have sodium hydroxide on its list of uses.
Since sodium formate is only slightly soluble in water, it harnesses the hygroscopic property.
This means that the sodium formate absorbs any moisture around it for easy manipulation.
This property is usually applied in manufacturing printed circuit boards and paper products (for better absorption).
How to spot sodium formate:
tell-tale signs of sodium formate are placed on products you see every day.
Sodium hydroxide might also be added into the formula to help increase its solubility levels.

Chemical properties of sodium formate include:
- Organic sodium compound
- White powder or flakes with sodium hydroxide crystals
- Often found in sodium formate solutions or sodium formate powder
Sodium Formate Applications
Aside from being used as an ingredient for sodium formate products, sodium formate has a variety of applications:
- Textile finishers use sodium formate to soften and/or remove excess water. Ferrous solutions are then used to help set dyes and brighten the fabric. This process is called dewatering.
- Extraction of formic acid. Aside from extracting, it helps in the formation of plastics that you see commonly around you today (hard plastics such as polypropylene). This is possible through the reaction between sodium chloride, caustic soda, sodium formate, and oil. – Sodium formate is also used to neutralize sodium hydroxide.
- Used as a cryoprotectant: sodium formate is added into ice cream to prevent it from becoming too hard in production.
- Used in sodium formate soap, sodium formate shampoo, sodium formate conditioner and sodium formate toothpaste.
- Sodium formate and sodium bicarbonate are also used as buffering solutions for manufacturing paper products.
- Sodium formate can be found in toothpaste.
The sodium hydroxide is responsible for converting food debris into simpler substances by breaking down enzymes that cause bad breath. When sodium hydroxide interacts with water present in your saliva, sodium carbonate and sodium bicarbonate are produced which acts as antiseptics good at killing bacteria that cause bad breath.
- Sodium formate is also used as a fire retardant. It can help slow down the fire from spreading quickly and thus giving you time to escape by itself.
- Sodium formate is also a cementing agent for sodium hydroxide and water.

Sodium Formate Solutions
The sodium formate solution that you can buy today has varying concentration levels. 97% of solutions are common in cleaning solution applications.
On the other hand, the weaker forms of sodium formates are most likely used in packaging or printing production.
Using sodium formate for chelation helps improve quality inkjet printing by preventing clogging and drying of ink nozzles on printers.
This gives you a clearer image during photo prints as well as text documents with accurate colors (not faded).
Is sodium formate a reducing agent?
Sodium formate is a sodium salt of formic acid; sodium formate hydrolyzes in the presence of water to sodium carbonate and HCOOH.
Thus it is an excellent reducing agent, converting into sodium carbonate, which is quite stable and not corrosive.
Sodium carbonate does not have any hazardous characteristics and could be used as a primary reducing agent.
It has been widely used in organic synthesis for example.
So you could use sodium formate directly if you want to buy sodium formate for lamination reactions or reduction of carbonyls such as ketones or esters to alcohols.
Is sodium formate toxic?
Sodium formate is corrosive and harmful to the eyes, skin, and respiratory system.
But sodium carbonate which is one of sodium formate’s final products has no toxicity, so sodium formate itself is not very toxic.

Is sodium formate explosive?
The answer is complicated because it depends on temperature and concentration.
When the molar ratio of sodium (III) ion/ sodium (II) ion in solution drops to 1:1, sodium metal precipitates out from its sodium salt solutions by a process called auto-decomposition.
Then another major issue appears: how do you separate this highly flammable white solid metal from an inflammable liquid phase containing HCOOH?
So when you want to buy sodium formate as sodium metal, you have to be extra careful about the sodium carbonate in the sodium formate solution.
It is best not to let sodium formate react with sodium carbonate in a strong base or any kind of oxidizer.
Is there sodium formate in sodium bicarbonate?
No. Sodium formate is an acid salt, while sodium bicarbonate (baking soda) is a basic salt, sodium hydroxide being the conjugate base of sodium carbonate (Na 2 CO 3).
They would react with one another to produce sodium hydrogen carbonate and water.
If you want to dissolve sodium metal by sodium carbonate solution, I recommend using liquid sodium bicarbonates or some other cheap chemical-grade sodium formates.
They are stable when heated to high temperatures and have low impurity contents.
Purity of sodium formate
Sodium formate is a sodium salt, so it contains sodium.
The purity of sodium formate should be more than 98%.
So when you select sodium formate, you must check the sodium content.
Sodium Formate Hydrate (NaHCOO) is sodium formate with hydrate, the sodium content is not more than 0.2%.
Sodium formate price and sodium formate supplier
Sodium Formate is widely used in the metal complex industry, dyeing and printing industry, leather tanning, and other industries, which means there are a lot of sodium formate suppliers now.
But since sodium formate is sodium salt, the sodium content should be controlled strictly, only a small number of sodium formate manufacturers can produce sodium formate with high purity and low sodium content.
This means that you should be careful when selecting sodium formate suppliers and manufacturers.
Sodium formate price is also related to sodium content, if sodium formate suppliers quote sodium formate price too low, you should think twice before you buy sodium from them.
How to buy sodium formate?
When you want to buy sodium formate, first check your project needs: what kind of grade sodium formate do you need?
Second, check sodium formate price and sodium formate supplier carefully.
If sodium content is too high, your project will be failed.
Finally, when sodium formate arrives at your factory or warehouse in large amounts, you should check sodium formate quality once again to make sure it meets the requirement of your project.

We also make and supply Polyacrylamide to overseas. Suneco Chem is one of the best Polyacrylamide Manufacturer in China.











